The Centers for Disease Control and Prevention continue to investigate a multi-state outbreak of fungal meningitis first announced on September 26, 2012. The outbreak originated with a preservative-free steroid injection distributed by the New England Compounding Center (NECC). Three lots of preservative-free methylprednisolone acetate, a form of injectable steroid, were recalled from the Compounding Center in Framingham, MA. The recalled lots were distributed to health care facilities, including many pain management and surgery centers, in 23 states. Investigators found the steroid injections contaminated by a rare fungus. Estimates indicate that approximately 14,000 patients may have received injections from one of the 3 lots of contaminated steroids. The affected patients include those who received injections in the back or in joints such as the hip, shoulder, elbow or knee.
The CDC is urging all of those who may have received an injection with a contaminated steroid to remain vigilant for signs of fungal meningitis or infection. Only those patients who received an injection in their back are at risk for fungal meningitis. According to the CDC’s investigation, the risk of developing fungal meningitis is highest within the first 6 weeks (42 days) after the last epidural or spinal injection with a contaminated steroid medication. Typically, symptoms appear within 1-4 weeks following the injection, but the onset of symptoms can vary according to reports.
Although more than 6 weeks has passed since the recall, the CDC recommends the best approach for patients is to continue to watch closely for the onset of symptoms consistent with meningitis for several months after the date of injection. Symptoms of fungal meningitis include new or worsening headache, fever, sensitivity to light, stiff neck, new weakness or numbness in any part of the body, slurred speech, and increased pain, redness or swelling at the injection site. It is important to remember that not everybody who received an injection will experience symptoms of meningitis or get sick. Whether an individual gets sick or not depends on several variables which are difficult to measure. Nevertheless, the CDC stresses that anybody who does experience symptoms of meningitis should seek medical care. For those who do not have symptoms and received their last injection with the contaminated steroid within the past 6 weeks, the CDC has advised healthcare clinicians consider performing a lumbar puncture. A lumbar puncture is a procedure during which a needle is placed inside a patient’s spinal canal in order to draw cerebral spinal fluid (CSF fluid). The specimen is sent to a laboratory for testing to confirm fungal meningitis.
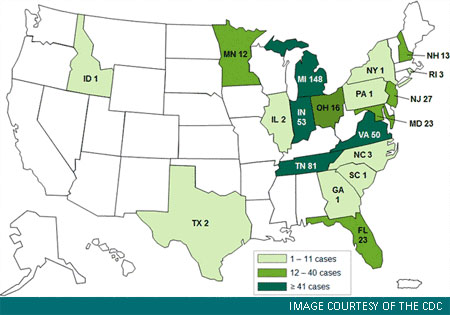
As of November 9, 2012, only one case of meningitis was reported in Pennsylvania. The highest number of cases reported thus far are in Michigan (128), Tennessee (81), and Indiana (52) (See CDC case count map). There are 428 total cases of fungal meningitis, stroke due to presumed fungal meningitis, or other infections affecting the central nervous system. In addition, 10 patients have suffered infections in a peripheral joint such as the knee, hip, shoulder or elbow.
Considering the estimated 14,000 patients who may have received injections with the contaminated medication, the number of those with diagnosed infections has remained relatively low. Still, those who received a contaminated injection and do not have any symptoms consistent with infection should remain alert for such symptoms. Also, if you or somebody you love recently received a pain injection but is unsure whether the injection was from a contaminated lot, the CDC website lists every health care facility that received a lot of the recalled methylprednisolone acetate from the NECC. Readers in Pennsylvania should know that the contaminated lots were recalled from only two facilities within the state: Allegheny Pain Management in Altoona, PA and South Hills Pain and Rehab Associates in Jefferson Hills, PA.
